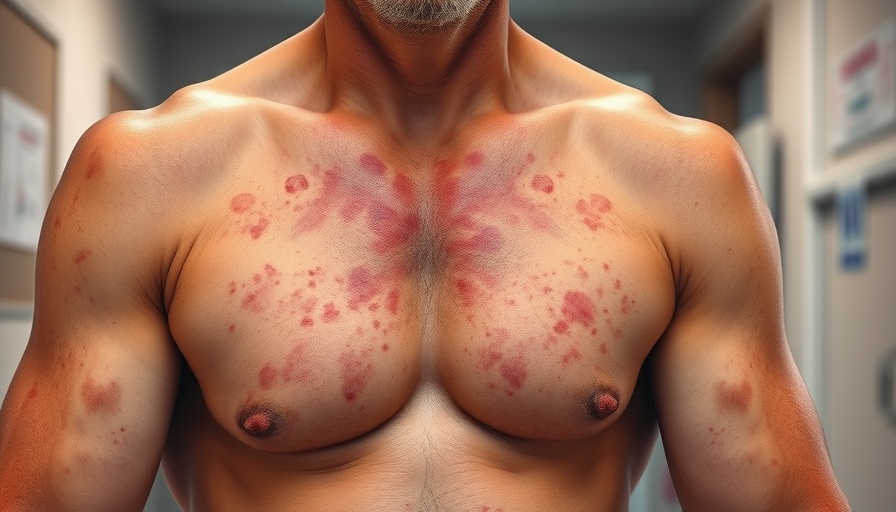
Uncovering a Rare Adverse Reaction to Omalizumab
Recently, a rare case report highlighted a significant yet often overlooked adverse event associated with the medication omalizumab. This monoclonal antibody, commonly prescribed for asthma and allergic conditions, has gained popularity due to its efficacy in managing symptoms for many patients. However, the emergence of a maculopapular eruption following treatment raises vital questions about patient safety and drug monitoring.
Understanding Maculopapular Eruptions
A maculopapular eruption is characterized by flat red areas on the skin that are often accompanied by raised bumps. Symptoms may vary in severity, leading to potential discomfort and distress for affected individuals. While many such eruptions are benign and resolve independently, they can indicate an underlying sensitivity to a medication. In this case, the patient experienced these symptoms shortly after initiating omalizumab treatment, prompting a reevaluation of the drug's safety profile.
Comparative Insights on Drug Reactions
This incident echoes findings from other recent reports on drug reactions, particularly within the allergy and immunology space. Following treatments with similar monoclonal antibodies, healthcare professionals have recorded rare skin reactions that underscore the necessity of vigilant patient monitoring. It's crucial for both physicians and patients to maintain transparency regarding potential side effects, so that they can make informed decisions about their treatment plans.
The Importance of Patient Communication
As medications like omalizumab become more commonplace, the dialogue between healthcare providers and patients cannot be overstressed. Patients should feel empowered to report any unusual symptoms, including skin eruptions. Timely communication can lead to prompt interventions, mitigating further complications associated with unexpected adverse drug reactions.
Insights on Safety in Drug Development
These incidents are a reminder of the complexities involved in drug development. Although clinical trials aim to identify and quantify potential side effects, some reactions only emerge once the drug is widely prescribed. The post-marketing phase is as critical as the pre-marketing phase, with ongoing surveillance needed to ensure medications remain safe for public use.
Future Considerations for Monoclonal Antibody Treatments
As we look forward, the consideration of rare adverse events will shape the future of monoclonal antibody treatments. Regulatory agencies are adapting their monitoring practices to implement more robust mechanisms that can swiftly identify such cases, further protecting patients' health. The balance between effective treatment and patient safety is vital in the evolving landscape of biopharmaceuticals.
Conclusion: Prioritizing Patient Safety
The case of the maculopapular eruption following omalizumab use highlights the need for greater awareness and proactive management of side effects. It serves as a vital reminder that even well-established medications can harbor unexpected risks. Patients are encouraged to engage with healthcare providers, remain vigilant about their health, and advocate for themselves in conversations about their treatment options.
 Add Row
Add Row  Add
Add 



Write A Comment