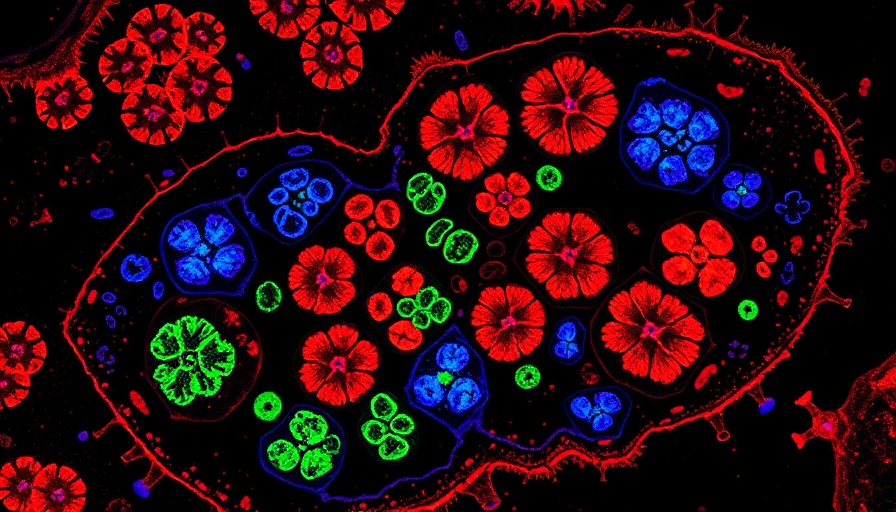
Revolutionizing Bone Therapy Through Jawbone Organoids
In a groundbreaking study led by Associate Professor Makoto Ikeya at Kyoto University, researchers have successfully developed jawbone-like organoids from induced pluripotent stem (iPS) cells. Published in Nature Biomedical Engineering, this research not only marks a significant advancement in biomedical engineering but also opens new avenues for understanding and treating various jawbone diseases.
The Challenge of Jawbone Health
The jawbone is the largest biomineralized organ in the human mouth and plays a crucial role in oral health and function. Unfortunately, jawbones are vulnerable to a multitude of factors that can lead to severe conditions, including genetic defects, infections, and physical trauma. Despite its importance, there has been a lack of effective models to study jawbone development and associated pathologies, which has hindered the advancement of therapeutic interventions.
Advancements in Organoid Technology
Ikeya and his team have laid down a solid foundation for a three-dimensional (3D) model of jawbone using iPS cells. Previously, they had developed a method to grow cranial neural crest cells (NCCs) in two-dimensional cultures. With their latest innovations, these NCCs were transformed into 3D jawbone organoids, enabling the exploration of complex tissue interactions that occur during jawbone development.
How Is This Research Conducted?
The research was meticulously designed. The team began with several iPS cell lines, including those derived from patients with osteogenesis imperfecta, a hereditary condition often referred to as brittle bone disease. By manipulating various cell signaling pathways, they could effectively coax these iPS cells into developing into specific jawbone tissues.
This involved stimulating NCCs into a precursor type known as mandibular prominence ectomesenchyme (mdEM). The researchers achieved this by combining specific molecular signals that mirror embryonic development, eventually yielding organoids that closely resemble the jawbone.
Modelling Bone Growth and Repair
Once these organoids were created, they were grown in conditions conducive to bone development, resulting in mineralized tissue structures that exhibit all the key traits of true bone. What’s remarkable here is the ability to transplant these organoids into immunocompromised mice, where they not only developed but integrated with the surrounding biological systems to form mature, vascularized bone tissues.
Potential Implications for Dental and Health Communities
The implications of this research stretch far and wide. With a robust model in place, researchers can explore the underlying mechanisms of jawbone diseases and work towards novel therapeutic approaches. This could transform the landscape of treatments for jawbone-related issues, making what once seemed impossible, quite feasible.
Future Directions and Outlook
The ongoing research is expected to delve deeper into the pathway of bone regeneration, evaluate the efficacy of these organoids in various disease models, and explore their application in regenerative medicine. The advancements in iPS cell technology also hint at promising prospects for overcoming traditional barriers in bone healing, which could foster innovations in both dental and orthopedic practices.
As we stand on the brink of this new frontier in biotechnology, the journey of understanding and treating complex disorders related to the jawbone becomes more tangible. This foundational research may very well pave the path towards a future where jawbone diseases are managed more effectively, improving the quality of life for countless individuals.
The importance of this study cannot be overstated, as it exemplifies the convergence of developmental biology, regenerative medicine, and innovative technology toward solving pressing health concerns.
 Add Row
Add Row  Add
Add 



Write A Comment